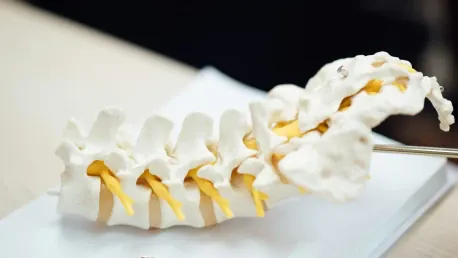
Acuity Surgical Devices, a veteran in delivering comprehensive spinal surgery solutions, has made a decisive move by investing in the AddUp FormUp 350 metal powder bed fusion system. This strategic shift aims to vertically integrate Acuity’s manufacturing processes, providing greater control over costs, lead times, and product quality. Since its inception in 2013, Acuity has developed a robust portfolio of products, including the Ventris ALIF Stand-Alone Anterior Lumbar System, the Tera-O Posterior Lumbar System, and the Stabilis NF Non-Fixated Anterior Cervical System. Each of these products has positioned Acuity as a reliable provider of advanced spinal surgery solutions, a reputation they are now looking to fortify with this new technological investment.
The decision to bring the FormUp 350 machine in-house marks a significant turning point for Acuity, which had previously depended on a global network of contract manufacturers. By incorporating the FormUp 350, Acuity aims to streamline operations and minimize reliance on external manufacturing partners. This transition is expected to elevate product quality and throughput mainly due to the advanced features of AddUp’s roller recoating system. Furthermore, the FormUp 350’s 350 x 350 x 350 mm build volume perfectly aligns with the demands for superior surface finishes and higher production capacities, enabling Acuity to better meet the increasing complexities of spinal surgery solutions.
Strategic Advantages of In-House Manufacturing
The primary advantage of this new integration is the level of quality and innovation it will bring to Acuity’s manufacturing processes. Bryan Cowan, co-founder and CEO of Acuity Surgical, has emphasized that this partnership with AddUp is a pivotal moment for the company. This strategic move will not only enhance the technological capabilities of Acuity but will also drive innovation and improve manufacturing quality. By having more control over the production parameters, Acuity can implement more iterative design processes, leading to faster design optimizations and overall better product performance.
One of the critical features of the AddUp FormUp 350 that Acuity found appealing is its efficient roller recoating system, which significantly improves the quality of the finished products. This aspect is crucial for producing medical devices with intricate geometries and stringent quality requirements. Acuity’s in-house manufacturing capabilities are expected to soar, especially considering AddUp’s proficiency in 3D printing technology. With the added advantage of an in-house system, Acuity can drastically reduce lead times and optimize production costs, making it more competitive in the high-stakes medical device market.
AddUp’s Role in Transforming Medical Device Manufacturing
Nick Estock, Deputy CEO of AddUp, has expressed immense excitement about supporting Acuity’s transition to in-house manufacturing by providing the FormUp 350. AddUp, known for its ISO 13485 certified Solution Center, has a strong foothold in the healthcare sector, underscoring its reliability and advanced technological prowess. The FormUp 350’s adoption by Acuity is more than just an upgrade; it symbolizes a commitment to stringent quality standards and continuous innovation. AddUp’s previous successes, such as gaining FDA 510(k) clearance for 3D printed Anatomical Great Toe through Anatomic Implants, further underscore the company’s capability to bring meaningful advancements to its partners.
This collaboration between Acuity and AddUp is anticipated to set a new benchmark in the medical device industry by fostering innovation and driving efficiency. The medical device sector has seen a growing trend towards in-house manufacturing, mainly driven by the need for better control over production parameters and quicker design iterations. With advancements in 3D printing technology, companies like Acuity can achieve enhanced surface finishes, precision, and throughput, ultimately leading to higher-quality products and better patient outcomes.
Conclusion: The Broader Impact on the Industry
Acuity Surgical Devices, a leader in spinal surgery solutions, has strategically invested in the AddUp FormUp 350 metal powder bed fusion system. This move is designed to vertically integrate Acuity’s manufacturing process, granting better control over costs, lead times, and product quality. Since its founding in 2013, Acuity has built a strong product portfolio, featuring the Ventris ALIF Stand-Alone Anterior Lumbar System, the Tera-O Posterior Lumbar System, and the Stabilis NF Non-Fixated Anterior Cervical System. These innovations have cemented Acuity’s reputation for providing top-notch spinal surgery solutions, a standing they intend to enhance with this new technological acquisition.
Bringing the FormUp 350 in-house signifies a major shift for Acuity, which had previously relied on global contract manufacturers. The addition of the FormUp 350 is expected to streamline operations and reduce reliance on external partners, boosting product quality and efficiency. The machine’s advanced roller recoating system and 350 x 350 x 350 mm build volume are perfectly suited to meet demands for high-quality surface finishes and increased production capacities, allowing Acuity to better address the evolving complexities of spinal surgery.