The medical device industry is undergoing a significant transformation with the advent of additive manufacturing (AM), commonly known as 3D printing. This technology is reshaping how medical devices are designed, produced, and utilized, offering unprecedented customization and efficiency. However, the journey to fully integrate 3D printing into this critical sector is fraught with challenges. This article explores the multifaceted impact of 3D printing on medical devices, drawing insights from industry experts and examining both the benefits and hurdles of this innovative technology.
The Power of Customization and Personalization
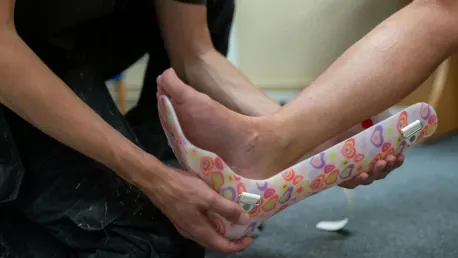
Additive manufacturing stands out for its ability to produce highly customized and personalized medical devices. Traditional manufacturing methods often rely on standardized sizes, which can limit the effectiveness of medical devices for individual patients. In contrast, 3D printing allows for the creation of devices tailored to the unique anatomical features of each patient. This level of customization is particularly beneficial in fields such as orthotics and orthopedics, where patient-specific solutions can significantly enhance treatment outcomes.
The process of creating personalized medical devices begins with detailed imaging of the patient’s anatomy, often using technologies like MRI or CT scans. These images are then converted into digital models, which serve as the blueprint for the 3D-printed device. This approach not only ensures a perfect fit but also improves patient comfort and satisfaction. Moreover, the ability to quickly produce prototypes and final products means that urgent patient needs can be met more efficiently, reducing lead times and improving overall care.
Advanced Design Capabilities and Complex Geometries
One of the most significant advantages of 3D printing in the medical device industry is its ability to produce complex geometries and intricate designs. Traditional manufacturing methods, such as CNC milling, often struggle with creating detailed structures, especially those with internal lattices or intricate patterns. Additive manufacturing, on the other hand, excels in this area, enabling the production of devices with enhanced performance attributes.
For instance, 3D printing can create lightweight yet strong structures that are ideal for implants and prosthetics. These complex designs can improve biocompatibility, reduce the weight of the device, and increase its strength and durability. Additionally, the design freedom offered by 3D printing facilitates innovation, allowing engineers and designers to experiment with new concepts and push the boundaries of what is possible in medical device development.
Regulatory Compliance and Material Consistency
Despite the numerous advantages, the adoption of 3D printing in the medical device industry is not without its challenges. One of the most significant hurdles is regulatory compliance. The medical field is highly regulated, and any new technology or material must undergo rigorous testing and approval processes. This is particularly challenging for additive manufacturing, as the materials and processes used are often new and lack historical precedent.
Experts like Victor Phan from Korthotics and Mateo Garcia from Restor3D highlight the complexities of navigating the regulatory landscape. Approved materials, such as titanium or PA11, in their powder form are viewed as new by regulators, complicating the approval process. Additionally, changes in material formulations by vendors can create compliance issues for manufacturers, further complicating the regulatory journey.
Geographical Constraints and Vendor Dependence
Another challenge faced by the medical device industry in adopting 3D printing is geographical constraints and vendor dependence. In regions like Australia, accessing support and materials for additive manufacturing can be difficult due to the centralization of manufacturers in the USA and Europe. This geographical disparity can lead to delays in obtaining necessary materials and support, hindering the efficiency and effectiveness of 3D printing operations.
Vendor dependence is another critical issue. Manufacturers rely on a steady supply of approved materials to maintain compliance and ensure the quality of their products. Any disruption in the supply chain or changes in material formulations can have significant implications for the production process. This dependence on external vendors underscores the need for robust supply chain management and strategic partnerships to mitigate risks and ensure continuity.
Educational and Awareness Efforts
For 3D printing to achieve its full potential in the medical device industry, there must be a concerted effort to educate stakeholders, including medical professionals and patients, about its benefits and processes. Both Korthotics and Restor3D emphasize the importance of knowledge transfer in promoting broader adoption and utilization of 3D printing technologies in medical settings.
Educational initiatives can take various forms, from workshops and training sessions for medical professionals to informational campaigns aimed at patients. By increasing awareness and understanding of 3D printing, stakeholders can make more informed decisions and embrace the technology with greater confidence. This, in turn, can drive innovation and improve patient outcomes, ultimately transforming the landscape of medical device manufacturing.
The Future of Additive Manufacturing in Medical Devices
The medical device industry is experiencing a profound transformation due to the emergence of additive manufacturing (AM), more commonly known as 3D printing. This innovative technology is revolutionizing the way medical devices are designed, manufactured, and used, enabling unprecedented levels of customization and efficiency. 3D printing allows for the production of complex, patient-specific devices that were previously impossible to achieve with traditional methods.
Despite the promising benefits, integrating 3D printing into the medical device sector comes with significant challenges. These challenges include stringent regulatory hurdles, ensuring consistent quality and reliability, and the need for substantial investment in new manufacturing processes and technologies. Moreover, there are concerns about the biocompatibility and long-term performance of 3D-printed medical devices, which must be addressed before this technology can be widely adopted.
This article delves into the impact of 3D printing on medical devices, providing insights from industry experts and examining both the advantages and the obstacles of this groundbreaking technology. By overcoming these challenges, the medical industry can fully leverage the potential of 3D printing to enhance patient care, reduce costs, and create medical solutions that were once beyond reach.