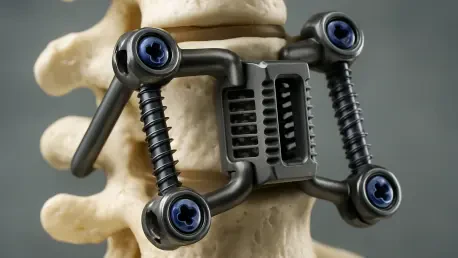
For millions suffering from debilitating back pain, the promise of relief through spinal surgery has long been tempered by the crude reality of the hardware involved—rigid, mass-produced metal implants that often create as many problems as they solve. This one-size-fits-all approach has been a fundamental limitation in spinal healthcare, forcing surgeons to adapt a patient’s unique anatomy to a generic device. However, a revolutionary development is poised to redefine the standard of care, leveraging a powerful combination of artificial intelligence, advanced biomaterials, and additive manufacturing. Nivalon Medical Technologies recently announced the successful production of its EvoFlex™ platform, the first spinal implant that is entirely patient-specific, motion-preserving, and completely free of metal, signaling a paradigm shift from standardized hardware to truly personalized medicine that could overcome the most persistent clinical challenges in spinal reconstruction.
A New Blueprint for Spinal Repair
The foundation of this groundbreaking approach lies in its AI-driven personalization, which completely discards the traditional model of off-the-shelf implants. The process initiates with a patient’s computed tomography (CT) scan, which provides a detailed three-dimensional map of their unique spinal anatomy. This data is then fed into a sophisticated digital design workflow powered by artificial intelligence. This platform empowers surgeons to conduct a virtual pre-operative simulation, allowing them to meticulously plan the procedure and design an implant with endplates that perfectly conform to the intricate topography of the individual’s vertebral bodies. Achieving such a precise anatomical fit is paramount for restoring the spine’s proper sagittal balance and ensuring correct facet joint alignment, factors that are critical for long-term success, especially in complex procedures involving multiple spinal levels where even minor inaccuracies can compromise the outcome.
At the heart of the EvoFlex™ implant is a fundamental rethinking of material science in spinal surgery, moving away from metals toward materials that work in harmony with the body. The device is constructed from two primary components, each serving a distinct but complementary purpose. The load-bearing structure is 3D printed from a proprietary Zirconia-Toughened Alumina (ZTA) ceramic, an advanced material chosen for its exceptional strength, superior biocompatibility, and bone-like mechanical properties. This entirely eliminates the host of complications associated with metal implants, such as corrosion, the release of metallic ions, and the damaging “stress shielding” effect. Furthermore, the ceramic is radiolucent, meaning it is invisible on MRI and CT scans, providing clinicians with an unobstructed view for post-operative monitoring. Sandwiched between these custom ceramic endplates is a flexible elastomeric core, engineered to mimic the biomechanical behavior of a natural intervertebral disc, restoring controlled, native-like motion to the spinal segment.
From Digital Design to Physical Reality
Transforming these intricate digital blueprints into tangible, life-changing devices is achieved through a strategic collaboration that harnesses the power of advanced additive manufacturing. Nivalon utilizes XJet’s NanoParticle Jetting™ (NPJ) technology, a sophisticated form of ceramic 3D printing that allows for the creation of the complex, load-bearing ZTA architecture with an exceptionally high degree of precision, reliability, and repeatability. This partnership with the Youngstown Business Incubator has been instrumental in establishing a scalable manufacturing pathway, successfully transitioning the EvoFlex™ platform from an innovative research concept into a clinically viable product ready for the next phase of evaluation. Independent analysis confirmed that the resulting 3D-printed ZTA represents a new and distinct microstructural class of biocompatible implant material, validating the novelty and efficacy of the manufacturing process itself and its ability to produce components that meet the stringent demands of medical applications.
Before any consideration for human use, the platform was subjected to a comprehensive battery of independent pre-clinical tests to validate its performance and safety across multiple domains. Biomechanical validation on a state-of-the-art spine simulator demonstrated that the implant exhibits stiffness curves and motion profiles that closely replicate those of a native human spinal segment, providing robust evidence of true motion preservation. Structural testing confirmed the implant’s incredible strength, showing it can withstand loads equivalent to over 3,200 pounds of force, validating its integrity under both normal and extreme loading conditions. Finally, biological validation studies showed that the ZTA ceramic surface actively supports mineral deposition and promotes biologically relevant ion interactions. This bone-like surface behavior indicates a strong potential for long-term, stable osseointegration, where the patient’s bone naturally grows onto and bonds with the implant, ensuring a durable and lasting fusion.
The Path to the Patient
The successful creation of the full-scale prototype marked a pivotal moment for the technology, signifying its transition from the research and development phase into scalable clinical manufacturing. This progress was built upon a solid intellectual property portfolio, including issued U.S. patents and several additional patents pending, which secured its innovative position in the market. With the pre-clinical validation complete and a clear manufacturing process established, the next steps involved advancing toward FDA Premarket Approval clinical trials. The company’s immediate focus turned to preparing for these crucial trials, with the first-in-human procedures scheduled to take place within the year. In a compelling testament to the profound belief in this transformative technology, the company’s co-founder, whose own spinal health challenges were the original inspiration for the mission, was slated to be among the first recipients of the implant.