The pharmaceutical packaging industry is on the cusp of significant changes as we move into 2024 and beyond, driven by innovations designed to enhance patient experience, ensure safety, and comply with evolving regulatory requirements. Dexter Tjoa, the CEO of Tjoapack, sheds light on the major developments set to shape the future of secondary pharmaceutical packaging. These advancements are more than just technical upgrades; they represent a fundamental shift in how medications are administered, tracked, and protected, aiming to offer a seamless and secure experience for patients and healthcare providers alike.
Pharmaceutical companies have long recognized the importance of patient-centric design in drug formulation and packaging. However, traditional methods, such as vials and syringes, come with significant limitations, particularly for injectable medications. These limitations have led the industry to explore more user-friendly solutions, ultimately transforming the way patients interact with their medications. As the sector innovates, the focus remains steadfast on improving safety and convenience while meeting stringent regulatory demands. This renewed emphasis on patient-centric approaches is pivotal in addressing the practical challenges patients often encounter during self-administration.
Enhancing Patient Experience
Patient-centric design is emerging as a crucial trend that is reshaping the pharmaceutical packaging landscape, focusing particularly on improving the administration process for patients. One of the most noteworthy advancements in this regard has been the shift towards pre-filled syringes (PFS). Historically, patients have struggled with self-administration, which often led to medication errors and contamination risks. These challenges have spurred the adoption of PFS, which offer precise dosing and minimize the likelihood of under- or over-dosing. This innovation not only ensures a safer and more convenient experience for patients but also reduces the complexities associated with traditional vial and syringe methods.
Pre-filled syringes are particularly beneficial for individuals who need to administer their medications at home, as they simplify the process significantly. As the healthcare industry continues to prioritize patient comfort and safety, it is likely that PFS will become even more prevalent. Moreover, the focus on patient experience extends beyond the ease of administration. Clear labeling and comprehensible instructions are equally vital, as they make medications simpler to use and understand. This is particularly crucial for oral solid dose (OSD) medications, where user confusion can lead to serious health risks. By prioritizing patient-centric designs, the pharmaceutical industry is taking significant steps to enhance the overall experience, ensuring that patients can manage their treatments with greater confidence and accuracy.
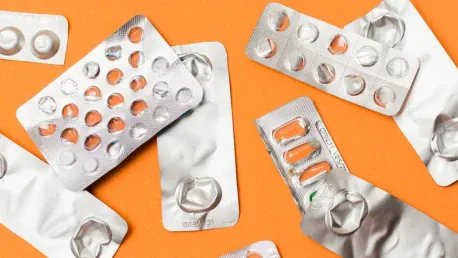
Optimizing Patient Safety
The threat of counterfeit drugs poses a significant risk to patient safety, and the statistics are alarming. In low- and middle-income countries, 13.6% of medicines are substandard or falsified, with this figure climbing to 19.1% for antimalarials. Governments worldwide have responded to this crisis by mandating serialization to enhance drug authenticity and traceability. Serialization frameworks like the EU Falsified Medicines Directive (FMD) and the U.S. Drug Supply Chain Security Act (DSCSA) have been instrumental in these efforts. These regulations require unique identifiers on drug packaging, allowing each product to be traced throughout the supply chain, thereby significantly reducing the risk of counterfeit medications reaching patients.
In addition to serialization, tamper-evident packaging plays a vital role in optimizing patient safety. Blister packs, for instance, are widely used in the EU to signal any unauthorized access or replacement of doses. By making tampering immediately noticeable, these packaging solutions help protect patients from compromised medications. The importance of these safety measures cannot be overstated, as they provide an additional layer of assurance that the medications reaching patients are authentic and safe to use. As the industry continues to evolve, it will be crucial to maintain and enhance these safety protocols to protect patients and uphold the integrity of the pharmaceutical supply chain.
Innovations in Secondary Packaging
Recent advancements in secondary packaging are further enhancing patient safety and convenience, offering innovative solutions that address both practical and regulatory needs. One such innovation is the development of kitting practices that incorporate additional materials like swabs, replacement needles, and user instructions into the packaging. This approach is particularly beneficial for pre-filled syringes (PFS), making them more user-friendly for patients with manual dexterity issues, such as the elderly. By integrating these supplementary materials, pharmaceutical companies can provide a more comprehensive and convenient experience for patients, thereby improving adherence to treatment regimens.
Smart labeling technologies are also emerging as a significant advancement in secondary packaging, designed to meet serialization demands and improve patient safety. Labels equipped with radio-frequency identification (RFID) or near-field communication (NFC) technology provide robust information and aid in regulatory compliance. These smart labels can track the conditions of temperature-sensitive drugs during transport, ensuring timely interventions to maintain drug quality. Additionally, there is a growing emphasis on child-proof and tamper-evident seals, with innovations in packaging seals and closures that enhance security and prevent unauthorized access. By making tampering readily visible, these solutions help mitigate risks, particularly among vulnerable populations, thereby ensuring a safer and more reliable pharmaceutical supply chain.
The Role of Contract Packaging Organizations (CPOs)
The pharmaceutical packaging industry stands at the brink of transformative changes as we head into 2024 and beyond, fueled by innovations aimed at enhancing patient experiences, ensuring safety, and adhering to evolving regulatory norms. Dexter Tjoa, CEO of Tjoapack, highlights key advancements poised to redefine the landscape of secondary pharmaceutical packaging. These aren’t just technical updates; they signify a fundamental shift in how medications are administered, monitored, and protected, focusing on providing a seamless and secure experience for both patients and healthcare providers.
Pharmaceutical companies have long understood the value of patient-centric design in drug formulation and packaging. Yet traditional methods, like vials and syringes, have distinct limitations, especially for injectable drugs. These constraints have driven the industry to seek more user-friendly solutions, revolutionizing how patients handle their medications. Ongoing innovations prioritize safety and convenience while meeting rigorous regulatory requirements. This renewed focus on patient-centered designs is essential in overcoming practical challenges faced by patients self-administering their treatments.