The groundbreaking promise of cell therapies to cure once-intractable diseases is increasingly colliding with the practical realities of complex and costly manufacturing, creating a significant barrier to widespread patient access. Innovative cell selection technologies are therefore becoming critical for unlocking the full potential of these treatments by improving efficiency, purity, and scalability. This analysis explores a disruptive microbubble technology, its real-world validation through strategic partnerships, and its projected impact on the future biopharmaceutical landscape.
The Shift Away from Traditional Methods
Addressing the Limitations of Magnetic Beads
The cell therapy market is experiencing unprecedented growth, with projections indicating a sustained surge in demand for scalable manufacturing solutions through 2028. This rapid expansion places immense pressure on existing production workflows, many of which rely on decades-old technologies that are ill-suited for modern, automated processes. The inherent limitations of these legacy methods are becoming increasingly apparent as the industry strives to move from clinical-scale production to commercial-scale availability.
At the heart of this challenge lies magnetic bead-based cell selection, a long-standing but problematic industry standard. This method is known to induce cellular stress, which can compromise the viability and therapeutic function of the final cell product. Furthermore, the potential for residual magnetic bead contamination and the complexities of multi-step separation and bead removal create significant workflow bottlenecks, hindering the efficiency required for GMP-compliant manufacturing and slowing the path to patient delivery.
Consequently, a clear trend is emerging toward gentler, bead-free alternatives. Industry analysis reveals a growing preference for technologies that minimize cell manipulation and eliminate foreign materials from the process. This shift is driven by the need to enhance the quality, potency, and safety of therapeutic cells, ensuring that the final product is as unperturbed and effective as possible. The move away from magnetic beads represents a pivotal evolution in biomanufacturing philosophy.
Bracco Imaging’s Zero-Footprint Innovation in Action
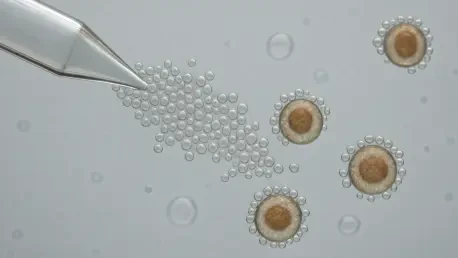
A prime example of this trend in action is the lipid-based microbubble technology introduced by Bracco Imaging. This platform offers a “zero-footprint” solution that directly addresses the shortcomings of magnetic beads. Instead of relying on persistent solid particles, the technology uses gas-filled microbubbles that can be functionalized to target specific cell populations, providing a gentle yet effective means of isolation.
The mechanism is elegantly simple: microbubbles act as a transient separation vector. After binding to target cells, they are used to float the desired population to the surface for collection. Once the selection is complete, the microbubbles are dissolved, leaving behind a clean, untouched cell population with no residual contaminants. This complete dissolution is the technology’s defining feature, ensuring the final product remains pure and free from process-related impurities.
This innovative approach supports both positive and negative cell selection strategies, as well as complex multi-step enrichment workflows. By providing researchers and manufacturers with a clean, unperturbed cell population, the technology is crucial for developing advanced therapies where cell quality is paramount. It represents a significant step toward simplifying upstream processing and improving the consistency of therapeutic cell manufacturing.
Expert Validation Through Strategic Collaboration
To accelerate the adoption of its microbubble platform, Bracco is pursuing key strategic collaborations that validate its alignment with next-generation manufacturing standards. A pivotal partnership with CellBri aims to co-develop a closed and automated cell selection system. This joint effort is designed to integrate the microbubble technology into a GMP-compliant workflow, reinforcing its readiness for clinical and commercial applications.
Further bolstering its credibility, Bracco has initiated a joint effort with the life science tools company Limula and the University of Fribourg. This expert-led initiative seeks to create a fully integrated and traceless cell processing platform. By combining Bracco’s dissolvable microbubbles with Limula’s automated processing technology, the collaboration aims to streamline the entire cell manufacturing process, from selection to final formulation, in a single, closed system.
These collaborations serve as powerful endorsements from key industry and academic players. They signal strong confidence in microbubble technology as a viable and superior alternative to traditional methods. By working with established experts in automation and cell processing, Bracco is not just developing a novel tool but is building an ecosystem around it, reinforcing its significance as a next-generation solution poised for widespread adoption.
The Future of Automated and Traceless Cell Selection
The adoption of microbubble technology holds the potential to dramatically streamline quality control and significantly improve manufacturing yields. By eliminating the need for bead removal steps and reducing cellular stress, the platform can shorten production timelines and lower the overall cost of cell therapies. These efficiencies are critical for making curative treatments more economically viable and accessible to a broader patient population.
The broader implications for the industry are profound. A gentler, traceless selection method enables the development of more complex and sensitive cell-based medicines that may be too fragile for harsh, bead-based processing. This could unlock new therapeutic avenues in areas like regenerative medicine and engineered T-cell therapies, where preserving the natural state of the cells is essential for efficacy.
Ultimately, the evolution of this trend points toward a future of fully automated, end-to-end manufacturing workflows. As technologies like dissolvable microbubbles become integrated into closed, continuous processing systems, the vision of “factory-in-a-box” cell therapy production moves closer to reality. Such advancements are poised to revolutionize the field, making curative therapies not only more powerful but also more affordable and consistently available.
Conclusion: A New Standard for Cell Purity and Viability
This analysis highlighted the clear limitations of traditional magnetic beads and presented dissolvable microbubble technology as an innovative solution poised to overcome critical manufacturing hurdles. The trend’s momentum is further evidenced by strategic collaborations that are driving its integration into automated, GMP-compliant workflows. These developments collectively address the urgent need for more efficient and scalable cell processing methods in a rapidly growing therapeutic sector.
The move toward “zero-footprint” technologies marks a significant paradigm shift in biomanufacturing. By prioritizing cell purity, viability, and process simplicity, these next-generation platforms are not merely incremental improvements but are foundational changes that will redefine industry standards. It became clear that such innovations are essential for unlocking the full therapeutic promise of cell-based medicines and ensuring they can be delivered to patients safely and reliably.